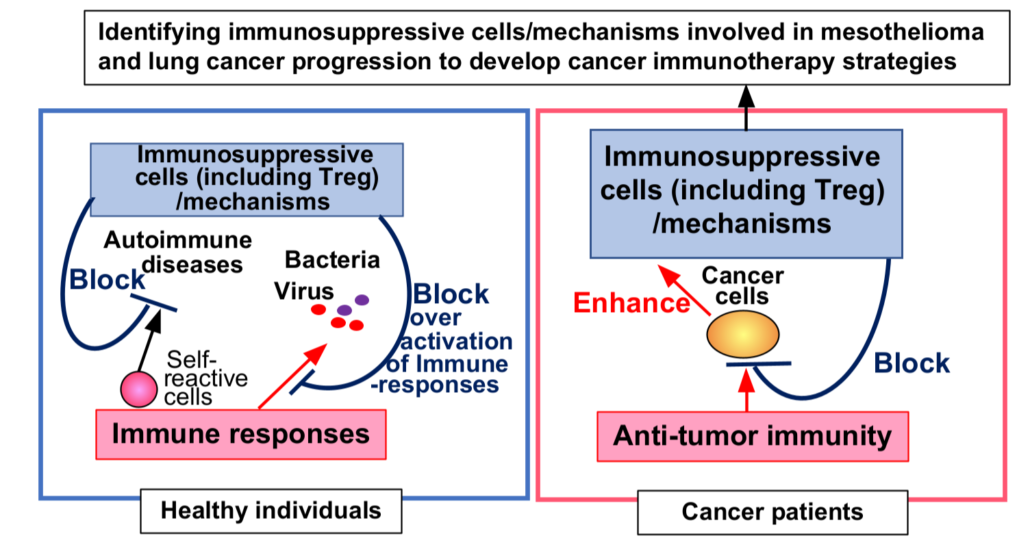
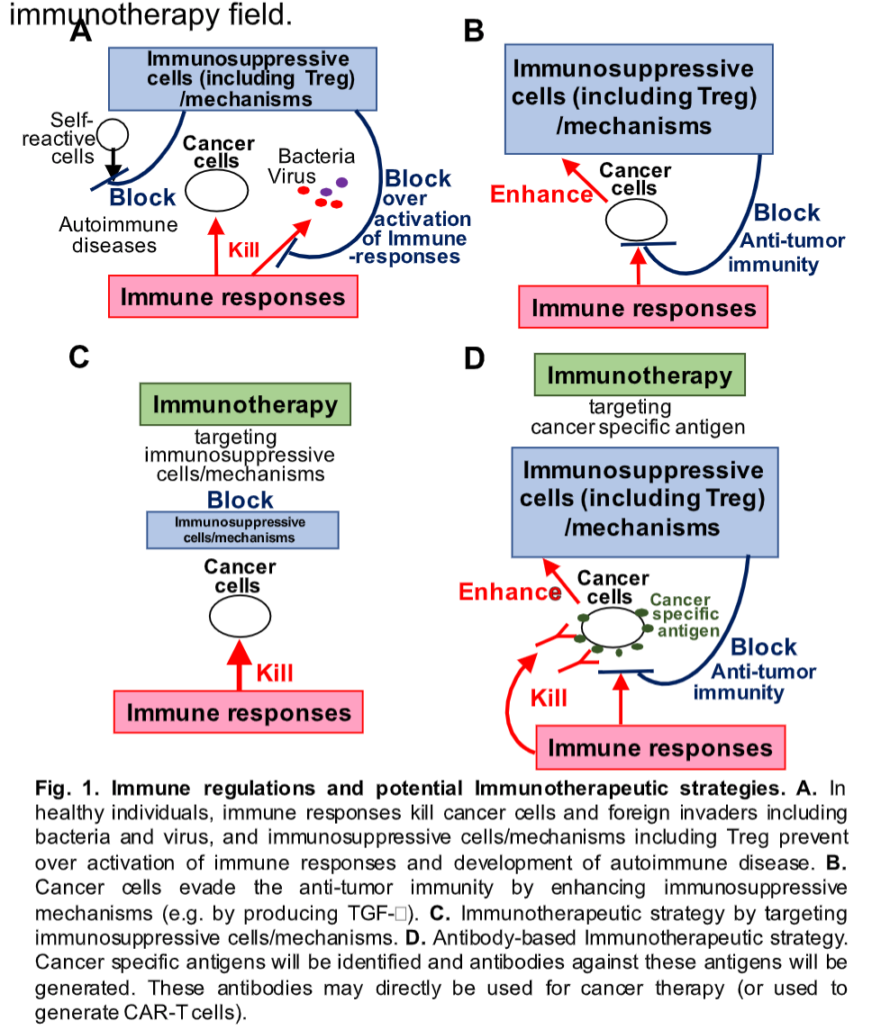
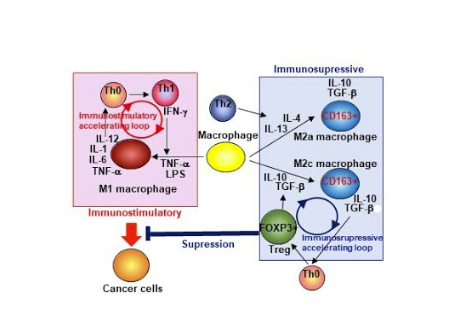
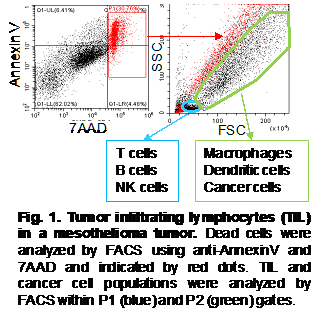
Researchers at PHLBI are working on developing cancer treatment strategies to treat a range of chest cancers including lung cancer, mesothelioma, thymus cancer, and esophogeal cancer. Learn what is happening in the lab in the below research updates.
December 2021
This month we will be submitting a publication on Contribution of Immunosuppressive Cells and Mechanisms in Developing Mesothelioma and Lung Cancer. This research could lead to a higher success rate for immunotherapy treatments. Immunosuppressive cells and mechanisms play important roles in modulating immune over-activation including preventing the development of autoimmune disease in normal individuals. Unfortunately, these immunosuppressive cells/mechanisms are utilized by cancer cells to evade anti-tumor immunity. Our research proposes a way of identifying these immunosuppressive cells/mechanisms utilized by mesothelioma and lung cancer cells. Then, we will develop strategies for blocking activities of the identified cells and the mechanisms to reactivate anti-tumor immunity in cancer patients, and these strategies can be used as new cancer immunotherapies.
September 2021
The aim of our research is to develop immunotherapy strategies for mesothelioma and lung cancer patients. To this end, we are identifying cancer therapeutic targets in these particular cancer patients. In order for us to identify these targets we needed to investigate how cancer developed in forty-one mesothelioma and twenty-one lung cancer patients. During this study, we made significant novel findings that in both cancers, immunosuppressive mechanisms are involved in cancer development, but in different ways for each cancer. This data was presented at iMig (The International Mesothelioma Interest Group Conference) in 2021. In addition, we are also preparing a manuscript detailing these findings for publication in a major cancer Journal.
Analyzing cancer and immune cells in tumors enables us to identify cancer specific antigens. If we identify said antigens, we can develop cancer immunotherapies strategies by utilizing CAR-T and monoclonal antibody technologies. Recently, DNA and mRNA vaccine technologies have dramatically improved due to the COVID-19 pandemic. By identifying cancer specific target antigens, we may be able to develop mesothelioma cancer vaccine strategies as well.
Analyzing Immunosuppressive Systems
Our immune system protects us not only from foreign invaders, such as bacteria and viruses, but also from cancer cells. However, some cancer cells utilize our immunosuppressive mechanisms and evade anti-tumor immunity. If we want to eradicate these cancer cells, we must enhance the anti-tumor immunity by blocking key immunosuppressive mechanisms in these cancer patients. To identify which immunosuppressive systems are exploited by cancer cells, we have analyzed immune and cancer cells in these tumors. We studied tumors from two different types of cancers, mesothelioma and lung cancer. Mesothelioma is a rare cancer (about 3,000 new cases per year in the US) that normally takes 20 to 50 years to develop. On the other hand, lung cancer is one of the most common (about 230,000 new cases per year in the US) and fastest growing cancers. Single suspension cells were prepared from forty-one mesothelioma and twenty-one lung cancer tumors. To assess anti-tumor immune responses in mesothelioma and lung cancer, we analyzed the T cell activation/proliferation in these tumors. Both CD4+ (helper) and CD8+ (cytotoxic) T cell populations were expanded in almost all tumors tested, suggesting that these T cells responded to these cancer cells. However, further analysis of T cell activation status also suggests that many of T cells in these tumors were inactivated and had lost their direct cancer-killing activities. To understand how these anti-tumor T cell activities were inactivated, we further investigated immunosuppressive FOXP3+ regulatory T cell (Treg) and M2-macrophage populations in mesothelioma and lung cancer tumors. These immunosuppressive cells play important roles in preventing autoimmune diseases in normal individuals. However, these cells are significantly increased during mesothelioma and lung cancer developments, suggesting that Treg and M2-macrophages inhibit anti-tumor immunity and contribute to cancer development. By blocking this immunosuppressive cell activity, we may be able to inhibit this cancer development.
We’ve observed that immunosuppressive Treg activity is inhibited by GITR (a modulator of immune response and inflammation) which demonstrates that it can be induced by its ligand and against anti-GITR antibodies. The anti-GITR antibody could effectively play a role in mesothelioma therapy. We also studied other immunosuppressive molecules including PD-1/PD-L1, CD39 and CD73 in these cancer patients. PD1/PD-L1 interaction inhibits anti-tumor T cell activity, and PD-1/PD-L1 immunotherapy was recently highlighted as an effective cancer treatment. In order to assess efficacy of PD-1/PD-L1 immunotherapy in mesothelioma patients, we have also analyzed PD-1 and PD-L1 expression levels and discovered that PD-1/PD-L1 was not a major immunosuppression mechanism in many of the mesothelioma patients tested. However, two immunosuppressive molecules, CD39 and CD73 seem to be involved in these cancer developments and potential targets for these cancer immunotherapies.
We are also investigating properties of Mesenchymal Stem Cells (MSC’s) for autoimmune, tissue repairing, and cancer therapies. In particular, we are studying MSC’s cell division and functions in order to better understand how we can expand functional MSC’s for cancer therapy.
December 2020
In the laboratory to date, we have demonstrated that immunosuppressive cells/mechanisms including regulatory T cells (Treg) are involved in mesothelioma cancer development, and we are also concentrating on identifying new therapeutic targets in mesothelioma tumors in order to develop new specifically targeted therapies for mesothelioma.
Mesothelioma and Thymoma Research
In developing our therapeutic strategies for mesothelioma and thymoma cancers, we are focusing on the immune responses in these isolated tumors at PHLBI. The immune system is not only an important defense system against foreign invaders including bacteria and viruses, but it also protects us from our own mutated cells even cancer cells. Our analysis of immune cell populations in mesothelioma and lung cancer tumors shows that immune cells, as expected, are activated and expanded to fight cancer cells in these tumors. Thus, these cancer cells are evading the anti-tumor immunity by using the immunosuppressive cells/mechanisms by inactivating these immune cells.
To this end, our analysis suggests that an immunosuppressive regulatory T cells (Treg) strongly contributes to mesothelioma cancer development. In healthy individuals, Treg plays an important role in preventing development of autoimmune diseases, but it also helps cancer development in mesothelioma patients. In fact, Treg deficient patients (IPEX patients) induce aggressive autoimmune diseases and die from serious cases. For example, immunosuppressive immunity, including Treg is necessary to block over activation of immune responses and prevent development of autoimmune diseases. Our immune system attacks foreign invaders, such as COVID-19 virus, by activating immune cells and inducing inflammation in tissues, including lung tissues. Typically, inflammation is reduced by Treg and other immunosuppressive mechanisms. However, if this immunosuppression fails, over activated immune responses induce severe inflammation and dysfunction of organs leading potentially to death.
These important immunosuppressive mechanisms that prevent auto immune dysfunction also aid in the ability of cancer cells survival by inhibiting the anti-tumor activity. However, this suggests that we can kill cancer cells by re-activating anti-tumor immunity through blocking these immunosuppressive mechanisms. A recently highlighted PD-1/PD-L1 therapy for mesothelioma has gained much attention, but our preliminary data of PD-1/PD-L1 therapies show that they are also ineffective due to low expression levels of PD-L1 in almost all mesothelioma patients tested. Thus, we still need additional new therapeutic strategies for mesothelioma patients and targeting Treg may be another more effective strategy for these mesothelioma patients. As we have previously suggested, inducing GITR signaling may be a potential therapeutic strategy since the development and function of Treg are inhibited by the GITR signaling.
In order to develop effective additional strategies, including antibody-based therapeutics for mesothelioma and thymoma patients, we will continue our analysis of tumors from our International Tissue Bank in order to identify anti-tumor antigens. Recent vaccine technologies, including RNA and DNA vaccines, have been dramatically improved as evidenced against the battle with COVID-19 virus. More importantly, these new vaccine technologies can also be applied to develop cancer vaccines for mesothelioma and thymoma. Thus, if we can identify these cancer specific antigens, we can then generate new RNA and/or DNA vaccines for these cancers as well. Even CAR-T immunotherapy also requires stable antibodies against tumor specific antigens. Therefore, while developing better mesothelioma and thymoma therapies, with these recently developed technologies, we will also be addressing a significant and unmet need of better antigen selection for the cancer immunotherapy field.
Even though the road is difficult, we are committed to accomplishing our mission, and we will continue to strive in our effort to advance rare cancer research, publish our new findings, and submit grants to the NIH, DOD and foundations to secure funding with our new findings.
December 2019
The Aim of PHLBI’s Research
The aim of our research is to develop Immunotherapy strategies for cancer patients including mesothelioma and lung cancer. Our immune cells seem to have the ability to recognize cancer cells as enemies and eliminate them. However, cancer cells are also cleverly evading the anti-tumor immunity by suppressing immune cells. This indicates that we can develop Cancer Immunotherapy strategies by backing the immunosuppressive mechanisms controlled by cancer cells. To this end, we are investigating what types of immunosuppressive mechanisms are utilized by cancer cells in mesothelioma and lung cancer patients. To evaluate the anti-tumor activities of the immune cells in tumors, we have been analyzing immune cell populations (TIL: tumor-infiltrating lymphocytes) and T cell (one of the key immune cells) activation status in mesothelioma and lung cancer patients.
So far, we have analyzed about 30 mesothelioma and 10 lung cancer tumors. We found that in 22 mesothelioma tumors out of 23 tumors, T cells seem to recognize tumor antigens and activated/expanded in these tumors. However, the tumor-killing activity of these T cells has already been deprived in these tumors. Activated T cells reach to several statuses such as effector, memory and exhausted T cells, and effector T cells can directly eliminate cancer cells. However, major T cell populations in all tumors tested are reached to memory or exhausted status, indicating that these T cells failed to eliminate cancer cells. T cells in these tumors seem to be inactivated by some mechanisms controlled by cancer cells. Since T cell activity is inhibited by signaling through several cell surface receptors including PD-1, T cells in mesothelioma tumors may also be inactivated by signaling through PD-1. To investigate this possibility, we have analyzed expression levels of PD-1 and its ligand PD-L1. PD-1 expression levels on T cells in mesothelioma tumors were high, but expression levels of PD-L1 on cancer and antigen-presenting cells (APC) are low in the majority of these tumors, suggesting that PD-1/PD-L1 is not key players to suppress anti-tumor immunity in these mesothelioma patients. Since PD-1/PD-L1 immunotherapy blocks the interaction of PD-1/PD-L1 to maintain anti-tumor T cell activity, this immunotherapy strategy may be not very effective for PD-L1 low mesothelioma patients.
Anti-Tumor Immunity in Mesothelioma Patients
We also considered the contribution of immunosuppressive T cell (regulatory T cells, Treg) and macrophages (M2-macrophage) to inactivate anti-tumor immunity in mesothelioma patients. As shown in Figure 1, anti-tumor immunity is regulated by the cooperation of T cells (CD4+ and CD8+ T cells, Th0 and Th1 cells in Figure 1 are CD4+ T cell subsets) and APC including macrophages and dendritic cells. As shown in the pink panel in Figure 1, macrophages are differentiated to M1-macrophages with IFN-g, and produce IL-12. Th0 cells are differentiated to Th1 cells with this IL-12, express IFN-g, and further activate APCs and cytotoxic CD8+ T cells (T cell subset has the ability to kill cancer cells) that can directly eliminate cancer cells. Therefore, anti-tumor activity is enhanced by this immunostimulatory accelerating loop (Figure 1). However, macrophages are also differentiated to immunosuppressive CD163+ M2-macrophages with cytokines (IL4, IL-13, IL-10, and TGF-b) as shown in the blue panel in Figure 1. These cytokines are also expressed by some of the cancer cells. Importantly, the M2-macrophages express immunosuppressive cytokines including IL-10 and TGF-b and suppress anti-tumor immunity. Furthermore, TGF-b induces differentiation of a strong immunosuppressive CD4+ T cell subset, Treg. Then, This Treg also expresses immunosuppressive cytokines TGF-b and IL-10 and these cytokines generate more M2-macrophages. Therefore, as shown in Figure 1 (Blue panel), the immunosuppressive lymphocyte populations are also enhanced by the immunosuppressive accelerating loop. Enhanced immunosuppressive environment generated by Treg and M2-macrophages may inhibit anti-tumor activity in mesothelioma patients. To investigate this possibility, we have analyzed Treg and M2-macrophage populations in mesothelioma tumors. Indeed, these immunosuppressive cell populations are increased in late-stage mesothelioma patients. Treg and M2-macrophages are potential targets for mesothelioma immunotherapy.
June 2019
Investigating Mesothelioma Tumor’s Immune Cells
Normally a person’s immune cells can recognize cancer cells as abnormal and eliminate them. However, some cancer cells can escape anti-tumor immunity, allowing them to survive and proliferate as malignant cancer. To investigate how cancer cells are evading anti-tumor immunity, we have been analyzing populations and activities of immune cells in tumors from mesothelioma and lung cancer patients. Particularly, we have focused on two immune cells, T cells and macrophages because both lymphocytes have two conflict subtypes, anti-tumor (effector T cells and M1 macrophages) and anti-tumor suppressive (regulatory T cells and M2 macrophages) populations. So far, we have analyzed 23 mesothelioma and 10 lung cancer cells. Importantly, T cell activation and proliferation are dependent on antigen recognition via T cell receptor (TCR) signaling. Therefore, if T cells are expanded in these tumors, these T cells could recognize tumor antigens. However, even though the T cells responded to tumor antigens, these T cells possibly reached memory/exhausted status and/or were inactivated via signaling through immunosuppressive receptors such as PD-1 and CTLA4. Therefore, to understand the regulation of immune responses in mesothelioma and lung cancer, first, we focused on T cell populations in tumors isolated from a different stage of cancer patients.
PD-1 and PD-L1 Therapy
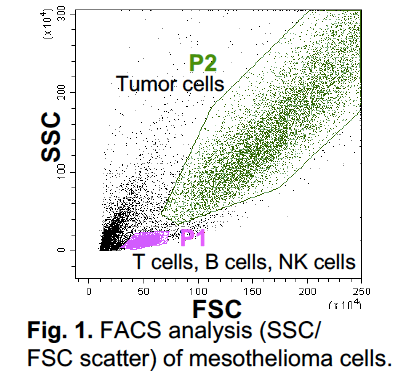
As shown in Fig. 1, tumor cells including tumor-infiltrating lymphocytes (TIL) were analyzed by FACS. Dead cell population was identified by AnnexinV and 7AAD staining. In the live cell aria in SSC/FSC scatter, we identify P1 and P2 populations (Fig. 1). CD4+ and CD8+ T cells, B cells, NK cells were detected in P1, and macrophages, dendritic cells, and cancer cells were detected in P2 (Fig. 1). However, in one tumor from mesothelioma and two tumors from lung cancer patients, we could not identify a clear P1 population, and both CD4+ and CD8+ T cell populations in these tumors are very low. In these patients, T cells seem not to be properly proliferated, suggesting no recognizable tumor antigens in these tumors. T cells in tumor cells are further analyzed using antibody against memory T cell marker CD45RO. Almost all CD4+ and CD8+ T cells in both tumors expressed CD45RO, indicating that these T cells have already reached to memory and/or exhausted status. This suggests that these T cells cannot eliminate cancer cells in these tumors. We also considered potential PD-1/PD-L1 immunotherapy in mesothelioma and lung cancers. To investigate this potential, we analyzed expression levels of PD1 on T cells and PD-L1 on cancer cells and antigen presenting cells (APC). Although many T cells expressed PD-1, expression levels of its ligand PD-L1 are poor in these cancers. Only in five tumors, we detected certain levels of PD-L1 expression. This suggests that PD-1/PD-L1 therapy seems not to be effective for the rest of 28 patients due to lack of ligand PD-L1 (PD-L1 low and negative). Currently, we are analyzing Immunosuppressive lymphocytes Treg and M2 macrophage populations in these tumors.